by E. Mironchik-Frankenberg, DVM.
Abbreviations: THC-Tetrahydrocannabinol, CBD-Cannabidiol, ECS- Endocannabinoid system, TCM-Traditional Chinese Medicine, GRAS-Generally recognized as safe, BBB-Blood-Brain-Barrier, NIH-National Institutes of Health, CB-Cannabinoid, CBR1/CBR2-Cannabinoid Receptor 1 or 2, OTC-Over the counter, PGE-1-Prostaglandin E1
Introduction:
In the natural world, our senses are constantly stimulated by the sights, sounds, colors, and aromas of the plant and animal species surrounding us. All living things use their senses to interact and communicate with each other and the environment around them. In the plant kingdom, and for many sessile or slowly moving species, a vital method of communication is via pheromones or “aromatics.” This is accomplished by the natural use of terpenes. All living organisms manufacture terpenes for certain essential physiological functions.6
Terpenes are the largest class of phytochemicals and are ubiquitous in the natural world.6,7,8 There are at least 20,000 different terpenes in existence4 and they play an important role in both kingdoms; flora and fauna. Physiologically, they are responsible for the protection of plants from predators, environmental stresses, pathogens, and diseases. They are also instrumental in intra and interspecies communication, such as alerting other plants in the vicinity to the presence of herbivores or attracting pollinating insects. In addition, they act as building blocks for more complex molecules, such as steroids, hormones, and cannabinoids.1,2,4,6,12
This broad group of aromatic, organic hydrocarbons are classified into families according to the number of repeating units of 5-carbon building blocks, called isoprene units, the structural hallmark of all terpenoid compounds.8 Examples of families include monoterpenes with 10 carbons, sesquiterpenes with 15 carbons, and triterpenes derived from a 30-carbon skeleton.7
When referring to these phytochemicals, the words terpene and terpenoid are increasingly used interchangeably, but the terms have slightly different meanings. The main difference between them is that terpenes are naturally occurring hydrocarbons,10 the only elements present are carbon and hydrogen. Terpenoids have been denatured by oxidation or chemically modified.1 Essentially, terpenoids are compounds related to, derivatives of, or modified terpenes.9A
Historical Uses:
Throughout history, various terpenes have long been used in folk medicine and cultural rituals worldwide.
Ancient healing arts, such as Traditional Chinese Medicine (TCM) and Ayurvedic medicine of India, utilized the therapeutic properties of terpenes in medicinal plants and herbs, long before their discovery by modern science.10 Many of these practices are still employed today. In fact, almost three-fourths of plant-based drugs were created based on the knowledge of folk medicine.10
In modern times, because terpenes form the major constituent of essential oils from plants, they are the basis of aromatherapy.10 They are also extensively used in flavorings, fragrances, and cosmetics.1,12
Terpenes are also common ingredients in the human diet and have generally been recognized as safe (GRAS) to consume by the US Food and Drug Administration.8,9
Modern Medicinal Use of Terpenes:
Scientific research has revealed much information about this class of physiologically active phytochemicals. Terpenes are lipophilic compounds that easily cross membranes and the blood-brain-barrier (BBB). They are pharmacologically versatile in their activities and can interact with cell membranes, neuronal and muscle ion channels, neurotransmitter receptors, G-protein coupled (odorant) receptors, second messenger systems, and enzymes.7,9 Some terpenoids act as serotonin uptake inhibitors (as does Prozac®), enhance norepinephrine activity (as do tricyclic antidepressants), increase dopamine activity (as do monoamine oxidase inhibitors and bupropion), and augment GABA (as do baclofen and the benzodiazepines).16 In addition, strong serotonin activity at the 5-HT1A and 5-HT2a receptors has been demonstrated.16
There is growing excitement and momentum surrounding the investigation into the use of terpenes for a wide range of medicinal benefits, including their anti-bacterial, anti-plasmodial, anti-viral, anti-diabetic, anti-depressant, and anti-cancer effects.10
One stunning example is paclitaxel, one of the most successful terpenes available in the market today, which has been included on the World Health Organization’s List of Essential Medicines.15 It is derived from the bark of the Pacific yew tree (Taxus brevifolia), originally used in TCM for treatment of different types of cancers.10 This terpene, original trade name Taxol®, is the most well-known natural-sourced cancer drug in the United States.14 This highly valuable medicine, the effects of which are supported by the full range of pharmacological and clinical studies, is used in the treatment of breast, lung, and ovarian cancer, as well as Kaposi’s sarcoma.13,14
A few of the more common sources of medicinal terpenes are: Melaleuca alternifolia (tea tree), Salvia lavandulifolia (Spanish sage), and Cannabis sativa.10
Terpenes in Cannabis:
These days, the word terpene has become strongly associated with cannabis. This is due to the buzz surrounding medicinal cannabis and its high terpene content. There are approximately 200 terpenes attributed to the cannabis plant.2
Many consumers do not realize that the terpenes, not the cannabinoids, are responsible for the aroma and flavor of cannabis. Cannabinoids themselves do not have any odor. Cannabis users have traditionally utilized the aroma of the plant to differentiate between ‘strains,’ now increasingly referred to as chemovars. Each chemovar is distinguishable by its unique combination of cannabinoids, terpenes, and flavonoids. Some refer to the terpene analysis of a chemovar as it’s “fingerprint.”8
In the cannabis plant, cannabinoids and terpenes share a common biosynthetic pathway. Geranyl pyrophosphate is formed as a precursor and is a parent compound to both cannabinoids and terpenes.8,9 These molecules are the oily compounds secreted in the glandular trichomes in the flowering tops of the plant, known as the ‘resin.’
Figure 1:
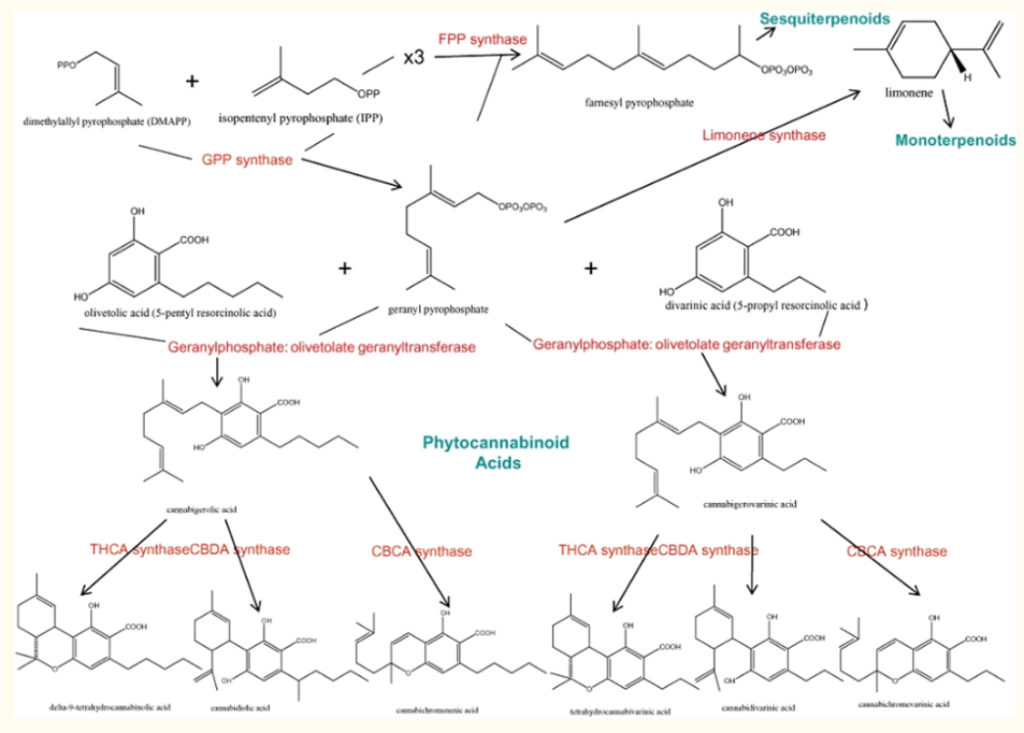
Terpenes are typically found at lower concentrations than cannabinoids, less than 1% dry weight of plant material,3 but they may represent 10% of trichome content.9 Nevertheless, they display unique therapeutic effects and may contribute meaningfully to the entourage effects of cannabis-based medicinal extracts.9
Terpenoid components in concentrations above 0.05% are considered of pharmacological interest.9 This was likely a contributing factor in the recent decision for the NIH to award nine universities federal grants totaling $3 million to study the “potential pain-relieving properties and mechanisms of actions of the diverse phytochemicals in cannabis, including both minor cannabinoids and terpenes.”5A
In the cannabis plant, monoterpenes usually predominate. However, the drying, processing, and storage of the raw plant material often drastically alters the composition of the terpene fraction, and usually results in a higher relative proportion of sesquiterpenoids, especially caryophyllene, in extracts.9
Regardless of the individual percentages, the most valuable cannabis product today is the terpene and cannabinoid-rich resin with its various pharmacological properties.13
Classes and Characteristics of Cannabis Terpenes:
As previously mentioned, there are different families of terpenes. The most common of the cannabis terpenes fall into the mono- and sesqui- terpene families, each with some prominent characteristics.
Monoterpenes: The smallest of the terpenes are monoterpenes. They contain the formula C10H16, are the most fragrant and volatile of the terpene classes and are known as the main component of essential oils. In addition, monoterpenes specifically are widely studied for their numerous pharmacological properties, especially their antiviral effects.10 Some of the most prevalent cannabis monoterpenes are listed below:
Myrcene(beta-myrcene). Myrcene is the most abundant terpene in cannabis and has many biologic activities:
- A prominent sedative,1,8 and combined with THC, may produce the ‘couch-lock’ phenomenon9
- Muscle relaxant8,9
- A potent analgesic1, acting at central sites that are antagonized by naloxone.7,8,9,16
- Anti-inflammatory,1,7,8 blocks the inflammatory activity of prostaglandin E2 9,16
- Hypnotic8
- Synergizes the antibiotic potency of other essential oil components against certain species of bacteria.16
- It inhibits cytochrome P450 2B1, an enzyme implicated in the metabolic activation of promutagens16
- Blocks the hepatocarcinogenic metabolite of Aflatoxin B11,9,16
- Lowers the resistance across the blood-brain-barrier, allowing itself and many other chemicals to cross the barrier easier and more quickly1
- Has been shown to increase the maximum saturation level of the CB1 receptor1
- Inhibitor of gastric and duodenal ulcers1
Also occurs in high concentrations in hops (Humulus lupulus) and lemongrass (Cymbopogon citratus),16 as well as in mangoes, eucalyptus, thyme.1
Pinene (alpha-pinene). Pinene is the most common terpene in the natural world. Its numerous physiologic attributes include:
- Anti-inflammatory9,10,16
- Bronchodilation8,9,16
- Inhibits the metabolic breakdown of acetylcholinesterase, a neurotransmitter that stimulates cognitive effects such as alertness and memory retention7,8,9,16
- Antibiotic activity10,16, including against MRSA9
- Antiseptic10
- Anti-cancer10,16
Also found in many other conifers, as well as in non-coniferous plants and some citrus fruit.1
Limonene. Limonene is the second most widely distributed terpene in nature. This is the precursor to other monoterpenoids. (see figure 1 above) It is highly absorbed by inhalation and quickly appears in the bloodstream,1,16 where it is readily bioavailable, rapidly metabolized, and may accumulate and be retained in adipose tissues.9 Its many effects include:
- Powerful anxiolytic 7,9
- Immuno-stimulating properties7
- Relieves heartburn and gastrointestinal reflux8,9
- Anti-convulsant8
- Anti-depressant16
- Anti-cancer effects,1,7,16 cytotoxic to cancer cells8,9,10
- Antimicrobial1,8,16
- Antifungal1,16 against dermatophytes,9 against Aspergillus16
- Inhibits production of Aflatoxin16
- Antioxidant, strong radical scavenging properties9
- Can dissolve gallstones8
- May have low-affinity interaction with cannabinoid receptors16
- Assists in the absorption of other terpenes through the skin/body tissues1
Major component of citrus rinds 1,16 also found in rosemary, juniper, and peppermint. 1
Linalool. Linalool has long been utilized as a pesticide, flavor agent, and scent, and is approved by the Environmental Protection Agency for these uses.1 In addition, it is a critical precursor in the formation of Vitamin E.1 It’s many pharmacological uses include:
- Analgesic7
- Anti-anxiety7,8,9,16
- Anti-inflammatory 1,7
- Powerful anticonvulsant7,8
- Anti-depressant16 via activity at serotonin receptors8
- Mediates stress8
- When applied topically, can heal acne/skin burns without scarring8,9
- Sedating in mice models9,16
- Local anesthetic effects (equal to procaine and menthol)9
- Anti-nociceptive at high doses in mice9
- Modulates glutamate and GABA neurotransmitter systems9
- Anti-leishmanial9
- Boosts the immune system1
- Reduces lung inflammation1
Also in lavender (Lavandula spp.),rose (Rosa spp.), and neroli oil (from Citrus aurantium)16 as well as hundreds of different plants and herbs.1
Terpinolene. This less studied terpene also has some important pharmacological properties:
- Sedative16
- Antimicrobial against a wide range of pathogens16
- Anti-malarial 16
- A central nervous system depressant1
- Anti-cancer effects.1
Also found in sage and rosemary 1
Sesquiterpenes: Sesquiterpenes, containing the chemical formula C15H24, are much larger compounds than monoterpenes and are much more stable in comparison.10 Some of the most common cannabis sesquiterpenes are listed below:
Caryophyllene (beta). Beta-Caryophyllene is the most common sesquiterpenoid encountered in cannabis.9,16 It is frequently the predominant terpenoid overall in cannabis extracts, particularly if they have been processed under heat for decarboxylation.9 It is also commonly referred to as “the dietary cannabinoid,” because of its unique ability to interact directly with the endocannabinoid system (ECS) and because it is found in so many vegetables. Its numerous physiological effects include:
- Potent antiinflammatory,7 via PGE-1,(comparable in potency to phenylbutazone)16
- Gastric cytoprotective activities, 7 without influencing gastric acid or pepsin secretion16
- It selectively binds to the CB2 receptor and is a functional CB2 agonist1,7,16
- Anti-malarial9
- May prevent nephrotoxicity1
Found in green leafy vegetables, black pepper, oregano, Copaiba balsam, cloves, and cinnamon leaves.1
Nerolidol. This non-toxic and non-sensitizing9 terpene has the following biological effects:
- Sedative8,9
- Skin penetrant that increases permeability and potentially facilitates cannabinoid absorption when applied topically for pain or skin conditions. 8
- Diminished experimentally induced formation of colon adenomas in rats, effective agent for enhancing skin penetration of 5-fluorouracil9
- Fungal growth inhibitor. 9
- Anti-protozoal parasite control benefits 9
- Potent antimalarial 9
- Anti-leishmanial agent9
Also found in neroli, ginger, jasmine, lavender, tea tree, and lemongrass.18
Caryophyllene oxide. This terpene is also non-toxic and non-sensitizing. Furthermore, it is the component responsible for cannabis identification by drug sniffing dogs.9 Biological effects include:
- Antifungal efficacy in a model of clinical onychomycosis comparable to ciclopirox olamine and sulconazole. 9
- Anti-platelet aggregation properties in vitro.9
Found in lemon balm, eucalyptus. 9
Humulene. This earthy, musky terpene is similar in structure to beta-caryophyllene and is sometimes referred to as alpha-caryophyllene.1 It also has several biological properties:
- Anti-tumor.1
- Anti-bacterial.1
- Anti-inflammatory.1
- Anorectic (suppresses appetite).1
Found in hops (Humulus spp), Vietnamese coriander,1 sage, and ginseng.
Evidence Supporting the Efficacy of Terpenes (and the Entourage Effect):
There is a rapidly growing body of scientific evidence supporting the added benefit of cannabinoid medicines that utilize the whole plant. Medicinal extracts that contain all the physiologically active components of cannabis include the various cannabinoids, terpenoids, and flavonoids. The entourage effect, a concept first postulated in 1998 by a group of researchers, including Dr. Raphael Mechoulam, has evolved to describe the notion that the therapeutic impact of the whole plant is greater than the sum of its individual molecular parts.
While still somewhat controversial, multiple studies highlight the important fact that differences exist in the overall effects seen between: A) using an isolated cannabinoid (i.e. THC or CBD), and B) using that same cannabinoid in a full spectrum cannabis extract.
For instance, in one often cited study,20 the researchers demonstrated the wider therapeutic window that was achieved when using a whole plant extract over purified CBD, concluding that “the higher efficiency of plant extract might be explained by additive or synergistic interactions between CBD and minor phytocannabinoids or non-cannabinoids presented in the extracts.”
In a 1974 study,17 3 different formulas of ‘marijuana’ were used to study certain effects in humans and several laboratory species. The researchers concluded that the effects seen were greater than expected, and that the potency of the samples they used could not be explained solely on the basis of their THC content alone. The authors postulated that synergistic action of other compounds within the samples were responsible.
Another study,21 was designed to determine whether there was any advantage (or disadvantage) to using the cannabis herb compared with isolated delta 9-THC. The authors concluded that the effects of their cannabis samples were not exclusively due to the THC. They supported the idea that the therapeutic use of cannabis has a greater potential than using pure THC alone. They also suggested that other non-psychoactive compounds might be discovered, and by selecting a suitable ratio of constituents it might also be possible to minimize side effects and increase efficacy.
Finally, a recent study2, conducted in Israel in 2018, tested the anti-inflammatory and anti-nociceptive properties of 3 different types of cannabis derived terpenoid oil preparations in vitro, and compared to CBD in vivo. The terpenoid rich cannabis extracts showed moderate anti-inflammatory activity without affecting TNF serum levels. They made several conclusions, including “in contrast to CBD that exerts prolonged immunosuppression and might be used in chronic inflammation, the terpenoids showed only a transient immunosuppression and might thus be used to relieve acute inflammation.” They also suggested that “the terpenoids exert their anti-inflammatory effects through a mechanism other than that employed by the cannabinoids.” In fact, another recently published study supported this idea.26
These, and other studies, have concluded that not all the therapeutic benefits of cannabis can be attributed to THC, or the cannabinoids alone, and lend support to the entourage effect concept.
Potential for Clinically Applicable Synergies in Veterinary Medicine:
We know that terpenes have beneficial uses in veterinary practice, as holistic practitioners and veterinary herbalists have been using them for centuries. These professionals maintain that whole herbs, or their extracts, are more efficacious and that the natural product contains other ingredients that act synergistically with the active principle, increasing the efficacy of the medicine.25
Similarly, when using whole plant cannabis medicine, the terpenes may increase effectiveness by modulating the cannabinoids and other components in various ways. For example, by binding with cannabinoid (CB) receptors, by sequestering THC or modulating its ability to bind to CB receptors, by altering the lipids surrounding CB receptors or the neuronal membranes themselves.16 In addition, they may have the ability to remodel G-proteins, alter the BBB, and/or act on other receptors and neurotransmitters.16 This is known as the “phytocannabinoid-terpenoid synergy,” a term coined by Ethan Russo.9
After all, the cannabis plant itself, due to its varying components, is considered polypharmaceutical. The dual status of beta-caryophyllene as both a terpenoid and a CB2 activator (like a phytocannabinoid) underscores the synergistic interplay between various components of the cannabis plant.8 This fact is further supported by a 2013 pain study23 demonstrating that other phytocannabinoids in combination, especially cannabidiol (CBD) and β-caryophyllene, delivered by the oral route appear to be “promising candidates for the treatment of chronic pain due to their high safety and low adverse effect profiles.”1,23
It has been suggested to tailor novel therapeutic treatments such as CBD-terpene extracts for use against numerous conditions including acne, MRSA, depression, anxiety, insomnia, dementia, and addiction.7 This concept can certainly be applied to veterinary therapeutics as well.
The potential exists for the creation of veterinary medicines using customized terpenoid and cannabinoid profiles, either with low levels of naturally occurring terpenes or with an enhanced terpene fraction. These formulas could be tailored for desired effects. For instance, consider the common complaint of anxiety issues in pets. CBD is a known anxiolytic. It has been suggested that the addition of anxiolytic terpenes, limonene and/or linalool, could contribute to the clinical efficacy of a CBD extract for this purpose.9
Other potential combinations to address specific conditions may include anti-inflammatory conditions (consider the combination of THC/CBD and myrcene), anti-convulsant therapies (the combination of CBD and linalool/beta-caryophyllene) or perhaps anti-cancer effects (adding limonene to various cannabinoid concentrations.) The possibilities, and the research opportunities, seem endless.
Currently, medicinal cannabis companies are increasingly employing the terpene fraction in their products and actively marketing this fact to consumers. However, cannabis products for veterinary use are slower to catch on. This may be due to numerous factors, including the expense involved in production and testing, as well as the lack of research into the effects on veterinary species. Currently, in the pet CBD market, the majority of OTC products contain only minimal amounts of terpenes, none at all, or are simply not tested for their presence. A recent study testing representative samples of OTC hemp-based pet products demonstrated this reality. The researchers found that the milligram quantity of total terpenes across these products ranged from 0.015–6.7 mg/mL or gram, with only 6 products with over 1 mg/mL or gram of total terpenes. Further examination for any single terpene at concentrations higher than 1 mg/mL revealed only three products that had a single terpene at that level.3 At this point, however, we do not know the ideal concentrations or percentages of terpenes necessary for specific conditions in veterinary species. Clearly, there is much work to be done.
Conclusion:
It is now universally accepted that the health benefits of fruits, vegetables, and other plant foods are due to the synergy and/or interactions between the different bioactive compounds or other nutrients present in the whole food. Similarly, plant-based therapeutics, including cannabis, exert their pharmacological effects, in multiple species, via synergistic or antagonistic interactions between the various phytochemicals contained therein. 7
While there is still much research needed to elucidate all the mechanisms of action involved in the myriad terpenoid effects in different species in vivo, it is evident that the cannabis terpenes have enormous potential for medicinal benefit, both on their own, and when used in whole plant cannabis medicines.
The genetic manipulation and creative hybridization of the cannabis plant to design specific chemovars may be the future of medicinal cannabis research and development. This knowledge may then be utilized in the development of easily administered, highly bioavailable, non-psychoactive, non-toxic whole plant cannabis products that utilize the entourage effect. This may be the key to untold possibilities and novel approaches to plant-based medicines which may ultimately provide millions of veterinary patients safe and effective therapeutic options, for multiple scenarios, in the not-too-distant future.
References:
1. https://www.medicaljane.com/category/cannabis-classroom/terpenes/
2. Galily, R., Yekhtin, Z., Hanus, L. The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis and Cannabinoid Research. Volume 3.1, 2018 DOI: 10.1089/can.2018.0014 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6308289/
3. Wakshlag JJ, Cital S, Eaton SJ, Prussin R, Hudalla C. Cannabinoid, Terpene, and Heavy Metal Analysis of 29 Over-the-Counter Commercial Veterinary Hemp Supplements. Vet Med (Auckl). 2020;11:45-55
https://doi.org/10.2147/VMRR.S248712
4. https://www.analyticalcannabis.com/articles/the-difference-between-cannabinoids-and-terpenes-311502
5. https://www.sclabs.com/terpenes/
6. Gershenzon, J., Dudareva, N. The function of terpene natural products in the natural world. Nat Chem Biol 3, 408–414 (2007). https://doi.org/10.1038/nchembio.2007.5 https://www.nature.com/articles/nchembio.2007.5
7. Andre, C. M., Hausman, J. F., & Guerriero, G. (2016). Cannabis sativa: The Plant of the Thousand and One Molecules. Frontiers in plant science, 7, 19. doi:10.3389/fpls.2016.00019 https://www.researchgate.net/publication/293193768_Cannabis_sativa_The_Plant_of_the_Thousand_and_One_Molecules
8. https://www.projectcbd.org/science/what-are-terpenes
9. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects, Ethan B Russo. Br J Pharmacol. 2011 Aug; 163(7): 1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3165946/ https://www.researchgate.net/publication/51485891_Taming_THC_Potential_cannabis_synergy_and_phytocannabinoid-terpenoid_entourage_effects
9A.https://terpenoids.net/difference-between-terpenes-and-terpenoids/
10. Cox-Georgian D., Ramadoss N., Dona C., Basu C. (2019) Therapeutic and Medicinal Uses of Terpenes. In: Joshee N., Dhekney S., Parajuli P. (eds) Medicinal Plants. Springer, Cham. Pp 333-359. https://doi.org/10.1007/978-3-030-31269-5_15 https://link.springer.com/chapter/10.1007%2F978-3-030-31269-5_15
11. https://www.projectcbd.org/science/terpenes-and-entourage-effect
12. https://www.newworldencyclopedia.org/entry/Terpene
13. Booth, J. & Bohlmann, J. 2019. Terpenes in Cannabis sativa – From plant genome to humans. Plant Science 284, pp 67-72. doi: 10.1016/j.plantsci.2019.03.022 https://www.sciencedirect.com/science/article/pii/S0168945219301190
14. https://dtp.cancer.gov/timeline/flash/success_stories/S2_Taxol.htm
15. https://en.wikipedia.org/wiki/Paclitaxel
16. John M. McPartland DO, MS & Ethan B. Russo MD (2001) Cannabis and Cannabis Extracts, Journal of Cannabis Therapeutics, 1:3-4, 103-132, DOI: 10.1300/J175v01n03_08 https://www.researchgate.net/publication/228897917_Cannabis_and_cannabis_extracts_Greater_than_the_sum_of_their_parts
17. Carlini EA, Karniol IG, Renault PF, Schuster CR. Effects of marihuana in laboratory animals and in man. Br J Pharmacol. 1974;50:299–309. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1776629/pdf/brjpharm00536-0132.pdf
18. https://en.wikipedia.org/wiki/Nerolidol
19. https://www.ncbi.nlm.nih.gov/pubmed/9721036
20. Overcoming the Bell‐Shaped Dose‐Response of Cannabidiol by Using Cannabis Extract Enriched in Cannabidiol. Ruth Gallily, Zhannah Yekhtin, Lumír Ondřej Hanuš. Pharmacology & Pharmacy, 2015, 6, 75‐85. http://dx.doi.org/10.4236/pp.2015.62010
21. Wilkinson JD1, Whalley BJ, Baker D, Pryce G, Constanti A, Gibbons S, Williamson EM. Medicinal cannabis: is delta9-tetrahydrocannabinol necessary for all its effects? J Pharm Pharmacol. 2003 Dec;55(12):1687-94. DOI: 10.1211/0022357022304. https://www.researchgate.net/publication/8906325_Medicinal_cannabis_Is_D9-tetrahydrocannabinol_necessary_for_all_its_effects
22. Gonçalves, E. C. D., Baldasso, G. M., Bicca, M. A., Paes, R. S., Capasso, R., & Dutra, R. C. (2020). Terpenoids, Cannabimimetic Ligands, beyond the Cannabis Plant. Molecules, 25(7), 1567. doi:10.3390/molecules25071567 https://www.mdpi.com/1420-3049/25/7/1567/htm https://www.mdpi.com/1420-3049/25/7/1567?type=check_update&version=1
23. Fine, P. G., & Rosenfeld, M. J. (2013). The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides medical journal, 4(4), e0022. https://doi.org/10.5041/RMMJ.10129 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3820295/
24.https://www.ahvma.org/holistic-veterinary-therapies/
25. https://vcahospitals.com/know-your-pet/veterinary-herbal-therapy
26. Finlay DB, Sircombe KJ, Nimick M, Jones C and Glass M (2020) Terpenoids From Cannabis Do Not Mediate an Entourage Effect by Acting at Cannabinoid Receptors. Front. Pharmacol. 11:359. doi: 10.3389/fphar.2020.00359 https://www.frontiersin.org/articles/10.3389/fphar.2020.00359/full

