by E. Mironchik-Frankenberg, DVM.
There is rapidly growing interest in the potential therapeutic use of cannabis among veterinarians around the world.
Unfortunately, there is a profound lack of formal veterinary education on the endocannabinoid system (ECS) and its clinical manipulation, despite its discovery more than 30 years ago. This gap has left practitioners with a lack of knowledge, as well as comfort, in meeting the increasing demands placed on them by clients. One of the biggest concerns for clinicians revolves around dosing strategy: what’s safe, what’s effective, where to start?
In this respect, our human counterparts are decades ahead of us. Physicians that have been practicing cannabinoid medicine for years have accumulated a wealth of anecdotal evidence and experience. Despite this however, many will say that the learning curve was steep. “Dosing cannabis is unlike any therapeutic agent to which I was exposed in my medical training,” says Dustin Sulak, D.O., the director of Integr8 Health, which has served 18,000 medical cannabis patients at offices in Maine and Massachusetts. 1
Traditional dosing protocols for most veterinary drugs are developed in accordance with manufacturers’ pharmacokinetic and pharmacodynamic studies. This data is factored into the recommended dosing rate and interval for each indicated species and listed in our favorite formulary. In calculating doses for most drugs used in our patients, body weight is the main determining factor. In addition, most of our traditional veterinary pharmaceuticals follow a linear dosing curve; as dose increases, so does efficacy (up to a point), and the risk of side effects. However, for most herbal or plant-based medicines, and specifically cannabis, it’s not so simple.
Many more factors come into play when it comes to dosing strategies for cannabinoid medicine. One size does NOT fit all. Dose calculation simply using bodyweight combined with patient specific factors is insufficient. Every individual, whether human or animal, responds to cannabis differently. Therefore, for example, 2 dogs that are roughly the same size and breed may have drastically different responses. There has been much discussion about the influence of novel considerations such as brain weight, age, and underlying endocannabinoid tone. In addition, there are multiple cannabis specific factors to consider.
For instance, it’s known in the medical community that children under the age of 10 are much more tolerant of THC then their adult counterparts, despite their smaller size.3 Could this age-related resistance translate to veterinary patients as well?
Clearly, it’s much more complicated than traditional pharmaceutical dosing…
Factors to consider for the practical dosing of cannabis:
- Patient-centered dosing factors: such as species, breed, age, weight, medical conditions, other medications, history with cannabis.
- Product-centered dosing factors: such as isolate vs broad vs full spectrum, formulation, concentration, method of administration, given with or without food, other active ingredients, cost.
- Unique cannabis factors: such as the entourage effect, cannabinoid ratios, the biphasic effect, tolerance, lipophilicity, varying chemovars, plant quality, etc.
For the purpose of this article, discussion will be centered around the unique cannabis factors, with some insight into the importance of product choice.
Developing an individual dosing plan requires multiple steps:
- Pick the cannabinoid(s) indicated based on the pet’s condition.
Choosing a cannabis-based medicine involves knowledge about the different cannabinoids and their effects. This will determine which cannabinoids will be best suited to the patient’s condition. For instance, a pet suffering from severe pain would benefit from a different set of active ingredients than one who has a seizure disorder. The information below can help guide the clinician in choosing which cannabinoids may be most appropriate for different scenarios.
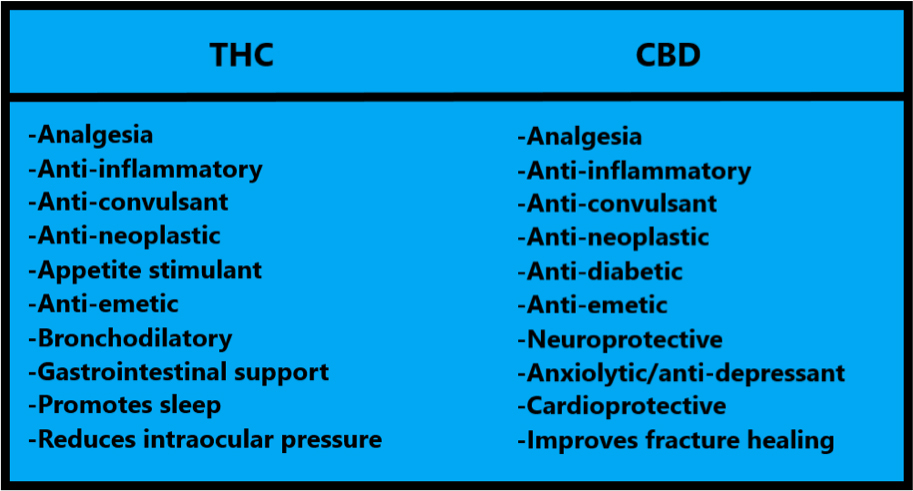
Source:14 Adapted from What all Veterinarians should know about Cannabis. (2019) Hazzah, T.
- Choose an appropriate product formulation.
After deciding on the ideal cannabinoid(s), choosing an appropriate product is necessary. While a detailed discussion of cannabis products is far beyond the scope of this article, a few important points will be highlighted.
There are many choices available and products can be derived either from hemp (widely available) or “marijuana”(available only through licensed dispensaries.) There are varied types of formulations, but can be separated into 2 main categories:
- Isolates contain a single, purified active ingredient, such as a CBD-infused MCT oil. These products, while concentrated in a single active ingredient, do not offer any entourage effect. Typically these types of products require higher doses than broad or full spectrum products.
- Full or broad-spectrum products contain all (full) or most (broad) of the cannabinoids, terpenes, and flavonoids present in the original plant. Many products geared towards veterinary patients, especially those derived from hemp, have had the THC removed and, by definition, cannot be considered “full spectrum.”
- A Ratio product is a standard or customized product with a specific ratio of desired cannabinoids, usually (but not always) CBD and THC, with or without the spectrum of other active compounds in the plant.
This illustrates one of the unique characteristics of cannabinoid medicine. While current research is largely focused on THC (tetrahydrocannabinol) and CBD (cannabidiol), the most well-known cannabinoids, there are over 140 of these compounds produced by the cannabis plant. Combining all the active components of the plant, the way nature intended, produces the “entourage effect.” This is the term used to describe the synergistic effect when using full spectrum or “whole plant” cannabis, the way that nature intended. Numerous studies have shown that increased benefit is obtained when using cannabinoid medicine in this manner, over choosing a single, isolated cannabinoid.2,3,4
If a ratio product is the appropriate choice, the graphic below illustrates the basic formula. Note that products such as these, with higher percentages of THC, will only be available in dispensaries.
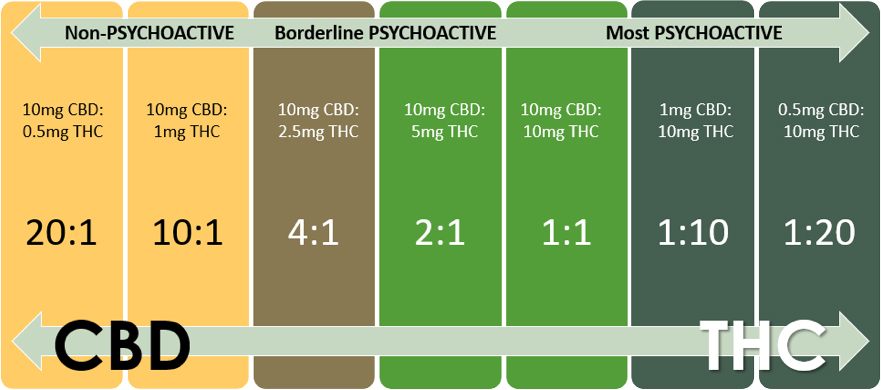
- Before choosing a starting point, consider these factors:
–Biphasic effect. An important factor to consider when determining a dosing protocol using cannabis, is the phenomenon known the biphasic dose-response curve. This is quite different than the traditional linear dosing curve. As the graph below demonstrates, “Biphasic” means that a low dose will generate one set of effects and possibly address one set of clinical issues, while a high dose will produce a second set of effects, and may address a different set of clinical issues.5 In many cases, the low dose and high dose effects are opposite. A good example of this is often seen when giving a patient a low dose of CBD results in stimulation, but a high dose leads to sedation.
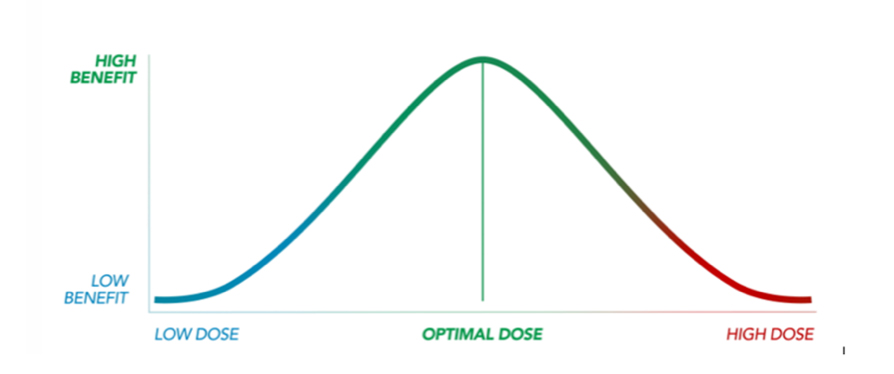
As Dr. Sulak explains, most patients have a certain threshold dosage of cannabis for their condition, below which they’ll actually experience a gradual increase in health benefits over time, and above which they’ll start building tolerance, experiencing diminishing benefits, and potentially more side effects.6 Therefore, finding the optimal dose for each individual is imperative, but can take time. Starting low and slowly escalating the dosage until beneficial effects are seen (without any adverse effects) is a vital step in the process.
–Tolerance: In addition, there is the phenomenon of tolerance. This is defined as the weakened response to cannabis due to chronic administration. For THC, repeated activation of CB1 receptors initially leads to desensitization, followed by internalization of the CB1 receptors from the cell’s surface. When this happens, higher doses are needed to achieve the desired effect.
This concept can be utilized when a patient’s condition necessitates the use of THC. This is especially important in dogs, since they are the species that is most susceptible to the effects of THC, due to the high numbers of CB1 receptors in the canine cerebellum.7 “Micro-dosing” is defined as using very small, subtherapeutic doses of cannabinoids to sensitize a patient (typically to THC). In the veterinary world, this is less than 0.5 mg/kg BID of cannabinoids,5 and in this author’s opinion, significantly less. This serves to reduce or eliminate the psychotropic effects of THC through the purposeful development of tolerance over a period of weeks. For veterinary patients, initially using the micro-dose strategy will facilitate the development of tolerance to the adverse effects of THC. Once this is achieved, THC dosages can be escalated to achieve the desired clinical benefit.5
- Determining a starting point.
What evidence exists to help make this decision?
There are numerous recently published studies (with more currently underway), to help guide clinicians. We have a growing body of evidence for dosing information, as well as some preliminary pharmacokinetic and safety studies. While we have much to learn, this new information can help eliminate some of the guesswork.
Before there was much evidence to guide pioneering clinicians, they would simply to start as low as possible, and slowly escalate. While this is certainly the safest method, it’s also the slowest and may lead to non-compliance and/or frustration on the part of the client.
An important fact to understand about the studies below, is that they were all done with proprietary formulations. Each of the test formulas has its own set of components and therefore, its own unique entourage effect. Most researchers are careful to point out this fact. Therefore, results obtained from one particular product may not necessarily directly correlate with those of another product. This is an inherent complication of cannabis-based medicine and the research surrounding it.
Summary of several recently published veterinary cannabinoid studies:
Since 2018, there has been a sharp increase in veterinary clinical studies on cannabinoids. In the US, due to the legal hurdles surrounding the schedule 1 status of THC, studies are performed using hemp-based products. Fortunately, we are seeing more trials using THC in companion animals originating from other countries.
-In one study published in 2018,8 dogs were used to determine the pharmacokinetics of a full spectrum CBD/CBDA dominant, hemp-based oil infused formula. The elimination half-life was 4.2 hours at both the 2mg/kg and 8 mg/kg dose, with no observable side effects. Then, a randomized, placebo-controlled, double blinded, cross-over study was conducted using the same formula at 2 mg/kg BID for 4 weeks. The results were positive showing efficacy for pain associated with osteoarthritis with no adverse side effects, although 9 out of 16 of the dogs did develop rises in ALP values on serum chemistry analysis.
-Another paper, also published in 2018,9 studied the pharmacokinetic profile of 3 different formulas given to healthy research dogs at doses of approximately 10 mg/kg and 20 mg/kg. Of the 3 different formulations, oral microencapsulated oil beads, oral CBD-infused oil, or CBD-infused transdermal cream, the infused oil had the greatest absorption and bioavailability, with a half-life of 3.33 +/- 0.9 hours for the 10 mg/kg dose and a half-life of 2.13 +/- 0.54 hours for 20 mg/kg dose.
-In this study completed at CSU in 2018,10 a randomized, blinded, placebo controlled clinical trial of 26 client-owned dogs with intractable epilepsy, a CBD-infused oil was given at 2.5 mg/kg po BID for 12 weeks, in addition to existing anti-epileptic medications (AEDs). 17 dogs completed the study, 9 in the CBD group and 7 in placebo group. Those in the treatment group had a significant reduction in seizure frequency (33% median reduction in mean monthly seizure frequency), with no significant difference in serum phenobarbital or bromide concentrations. 3 of the 12 dogs assigned to the CBD group had to be removed, 1 due to worsening condition and 2 that developed ataxia. Study dogs also developed increases in ALP, with unknown clinical significance, but no adverse behavioral effects were seen.
-Another recent study published in 201911 looked at single dose PK and safety of a hemp-based CBD product in healthy dogs and cats for 12 weeks. Both species were given 2 mg/kg BID, in either an oral canine whole-plant CBD-infused soft chew or an oral feline CBD-infused fish oil. Both had CBD and CBDA in a 1:1 ratio. It showed short pharmacokinetic half-lives of CBD in dogs (T ½ life mean was 1 h) and cats (T ½ life mean was 1.5 h), with cats showing far lower oral absorption kinetics or rapid elimination suggesting dosing may differ between the two species. Safety testing revealed no significant serum chemistry evaluations in dogs, and in the feline subjects, values were not observed to be outside of the normal ranges at any time point for the cats other than a single cat with elevated ALT level during treatment. Adverse effects in dogs were minimal, loose stool being the most common at 3.3%. In cats, the most common adverse effects were licking and head shaking after administration of the oil. The study concluded that overall, hemp-based CBD appears to be relatively safe in healthy populations of dogs and cats, and dogs appear to absorb CBD better than cats.
-An exciting, recently published study12 looked at the safety and tolerability of escalating doses of 3 different cannabis formulations: CBD, THC, and a ratio product containing both CBD and THC in a 1.5:1 ratio. This randomized, placebo controlled, blinded, parallel study tested the above formulas against 2 placebos in healthy dogs, and evaluated the occurrence and severity of adverse events (AEs) during a series of up to 10 increasing doses. The highest dose achieved in each category was ~62 mg/kg CBD in the 10th dose, ~49 mg/kg THC in the 10th dose, and ~12mg/kg CBD + 8 mg/kg THC in the ratio product in the 5th dose. The doses were increased by 2-2.5 times following the initial dose, and thereafter, serial doses increased by 1.2-2-fold. The escalation interval was every 3 days. The results also showed that AEs were reported in all groups, including placebo groups, with 94.9% being mild. In the CBD group, there were only mild AEs and the dose escalation was tolerated as well as placebo, with completion of all 10 doses. In the THC group, the majority of AEs were mild, with 2 reported moderate and 1 reported as severe, also with completion of all 10 doses. The CBD:THC ratio group had the highest percentage of AEs rated as severe and necessitated the discontinuation of dose escalation after the 5 doses. Only 2 dogs developed ALP elevations during this study, both deemed not clinically significant.
How can we use this information clinically?
So, how can we use the above information and what reasonable assumptions can we make for veterinary patients?
We know from the above studies that CBD is generally well-tolerated and side effects are minimal. The evidence also supports short half lives in dogs and cats, indicating at least twice daily dosing. The potential exists for increased liver enzymes in some patients and the significance of this is yet unknown.
When using any product containing THC, this should be considered the limiting factor when it comes to dosing and product selection. Adverse events from cannabis products mainly pertain to THC and are dose dependent. Higher doses of THC, especially when not titrated appropriately, are more likely to cause unwanted side effects.1 Combining cannabinoids can lead to enhanced effects.
In the dose escalation study above,12 keep in mind, the authors did not account for the tolerance that occurs with appropriate titrational dosing, when they chose a study design of q 3 days escalation. By not allowing enough time for tolerance to occur, we may not be getting an accurate clinical picture of THC dosing in dogs.
Specific recommended dosing strategies:
There are several published dosing recommendations available, authored by skilled veterinary cannabis specialists using their unique clinical experience and medicinal cannabinoid knowledge:
CBD Dosing:
Dr. Gary Richter, in an article published in May 2019, recommends 0.1-1.1 mg/kg CBD BID (calculated from the published 0.5 – 5 mg CBD per 10 pounds of body weight.) Start low and gradually increase every 4-7 days.13
In another soon to be published paper, Dr. Hazzah recommends starting CBD ~0.1mg/kg BID and increasing the dose (every 5-7 days) until efficacy or dysphoria is noted. She notes that most cannabis products are dosed orally at an interval of every 12 hours, however depending on the disease state, increased frequencies of every 6-8 hours or accelerated titration can be implemented. 14
From his book published in 2015, Dr. Silver discusses the variable effective doses of CBD ranging from the low end of 0.05 – 0.1 mg/kg up to 1-5 mg/kg BID (high end). 15
Overall, based on the above, a common recommendation for a starting point appears to be 0.1 mg/kg for CBD. However, regardless of the starting dose, remember that every patient is different. Often, doses nearer the lower end of the range can be effective, but higher doses may be necessary in certain circumstances.
THC or ratio product Dosing:
THC is always the limiting factor when dosing. Dr. Richter, in the same article, recommends that for THC consider 0.05-0.14 mg/kg BID (calculated from the published 0.2-0.6 mg per 10 pounds body weight.) Start low and slowly increase the dose every 4-7 days. Monitor closely for sedation, loss of balance, or loss of mental acuity. Decrease the dose or discontinue immediately if any side effects are seen. 13
Dr. Hazzah recommends starting THC significantly lower than 0.1 mg/kg BID for THC and slowly increasing the dose every 5-7 days until efficacy or dysphoria is noted. Depending on the disease state, increased frequencies or accelerated titration can be implemented.14
Dr. Silver, in his book, recommends that for THC dominant products, give 0.1- 0.25 mg/kg THC per day.15
Overall, these doses are in sync with the concept of “micro-dosing.” However, higher doses may be possible and in fact, necessary on a case-dependent basis.
Remember that ratio dosing can assist in the development of tolerance to THC through the initial use of a high CBD to low THC ratio (for example, a 20:1 CBD:THC ratio) to avoid toxicity while tolerance to the adverse effects of THC is being established. Once tolerance has been established, the dosage of the THC component can then be increased safely. This can be accomplished by several methods:
- Increasing the dose of the ratio product (which will equally increase doses of both cannabinoids.)
- Gradually switching to different ratio products with increasing amounts of the THC component (for example, switching to an 18:1 CBD:THC ratio, then 10:1, etc.)
- Using a combination of products to slowly increase the THC component (for example, giving the 20:1 product, but also adding increasing amounts of a THC dominant product to create a unique ratio.)
- By using custom formulations with appropriate increasing ratios of THC with each new bottle.
The general approach of “start low and go slow” is still a good rule to follow. However, we can now use the above evidence to better choose a starting point. In addition, for certain clinical conditions, there is growing evidence to approximate target doses or anticipated therapeutic endpoints. From the initial dose, gradual titration “until effect” (a concept veterinarians are familiar with), should be applied, with the understanding that the optimal dose as illustrated in the bell curve can vary remarkably between patients.
- Other tips to remember:
-Due to the highly lipophilic nature of cannabinoids, giving in a lipid-based formula (such as MCT oil) or with a small fatty meal may be beneficial 11
-CBD can be expensive, so being cost conscious with your dosing is warranted. The study on osteoarthritis pain in dogs showed that doses of both 2 mg/kg and 8 mg/kg were effective, with the higher dose having no advantage.8 Therefore, clearly using the smallest effective dose is warranted for multiple reasons, including cost and risk of side effects.
-Based on the studies above, it appears that healthy subjects are more tolerant to dosing and side effects. It may be prudent to start with a lower dose for patients that are older, have multiple conditions or in polypharmacy situations.
-Drug interactions. While this should always be considered, especially when combining with other sedatives, anticonvulsants or polypharmacy, “clinically, significant drug interactions have proven rare, and there is no drug that cannot be used with cannabis, if necessary.” 16
-Good patient monitoring via physical exams, periodic laboratory screening, especially for any liver enzyme elevation, and utilizing owner journals can prove invaluable.
Finally, it’s clear there is much work to be done. “Cannabis is unlike any other medicine due to its wide range of effective dosages, incredible safety profile, broad physiologic mechanisms of action, and versatility in treating a wide range of symptoms and diseases. Using the correct dose of cannabis is the single most important factor in having a successful therapeutic relationship with this powerful herb.”6 As more veterinary specific research is completed, we’ll be able to further refine our dosing decisions. However, it will be years before there are long-term safety profiles and thorough disease-specific studies in veterinary patients. Until then, clinicians, and the veterinary profession, must embrace the potential that exists in this emerging discipline. Only by promoting advancements in research into veterinary cannabinoid therapeutics, can we hope to offer the best treatment options to our clients and patients.
References:
- Russo, E. & Guy, G. (2006). A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Medical Hypotheses, 66, 234–246. doi:10.1016/j.mehy.2005.08.026
- Russo, Ethan B. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects, Br J Pharmacol. 2011 Aug; 163(7): 1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3165946/
- Ruth Gallily, Zhannah Yekhtin, Lumír Ondřej Hanuš. Overcoming the Bell‐Shaped Dose‐Response of Cannabidiol by Using Cannabis Extract Enriched in Cannabidiol. Pharmacology & Pharmacy, 2015, 6, 75‐85. http://dx.doi.org/10.4236/pp.2015.62010
- Hartsel, J., Boyar, K., Pham, A., Silver, R., & Makriyannis, A. (2019). Cannabis in Veterinary Medicine: Cannabinoid Therapies for Animals. In R. Gupta, A. Srivastava, & R. Lall, Nutraceuticals in Veterinary Medicine (pp. 121-155). Switzerland: Springer Nature . doi:10.1007/978-3-030-04624-8
- Herkenham, M., Lynn, A. B., Little, M. D., Johnson, M. R., Melvin, L. S., de Costa, B. R., & Rice, K. C. (1990). Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences of the United States of America, 87(5), 1932–1936. doi:10.1073/pnas.87.5.1932
- Gamble, Boesch, et al. Pharmacokinetics, Safety and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs. Frontiers in Veterinary Science, July 2018; Vol 5, article 165. https://www.frontiersin.org/articles/10.3389/fvets.2018.00165/full
- Lisa R. Bartner et al. Pharmacokinetics of cannabidiol administered by 3 delivery methods at 2 different dosages to healthy dogs. The Canadian Journal of Veterinary Research, 2018; 82: 178-183 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6038832/
- Stephanie McGrath, Lisa R. Bartner, Sangeeta Rao, Rebecca A. Packer, and Daniel L. Gustafson. Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy. Journal of the American Veterinary Medical Association, June 1, 2019, Vol. 254, No. 11 , Pages 1301-1308. (https://doi.org/10.2460/javma.254.11.1301)
- Kelly A. Deabold, Wayne S. Schwark, Lisa Wolf, and Joseph J. Wakshlag. Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats. Animals 2019, 9(10), 832; https://doi.org/10.3390/ani9100832. https://www.mdpi.com/2076-2615/9/10/832/htm
- Vaughn D, Kulpa J and Paulionis L. (2020) Preliminary Investigation of the Safety of Escalating Cannabinoid Doses in Healthy Dogs. Front. Vet. Sci. 7:51. doi: 10.3389/fvets.2020.00051 https://www.researchgate.net/publication/339188667_Preliminary_Investigation_of_the_Safety_of_Escalating_Cannabinoid_Doses_in_Healthy_Dogs
- Hazzah, Trina. (2019) What all veterinarians should know about cannabis. White paper. https://www.veterinarycannabis.org/uploads/1/1/6/0/116053487/white_paper__hazzah_.pdf
- Silver, R. (2015). Medical marijuana & your pet. Lulu publishing.
- MacCallum, C. & Russo, E. Practical considerations in medical cannabis administration and dosing. European Journal of Internal Medicine 49 · January 2018. DOI: 10.1016/j.ejim.2018.01.004 https://www.researchgate.net/publication/322257368_Practical_considerations_in_medical_cannabis_administration_and_dosing
- Goldstein, Bonni MD. 2016. Cannabis Revealed, How the world’s most misunderstood plant is healing everything from chronic pain to epilepsy.

