by E. Mironchik-Frankenberg, DVM.
Abbreviations- MDR: Multi drug resistance, ATP: Adenosine triphosphate, ABC: ATP binding cassette, P-gp: P-glycoprotein, BCRP: Breast cancer resistance protein, WSU: Washington State University, BBB: blood-brain-barrier, THC: Tetrahydrocannabinol, THC-COOH: Tetrahydrocannabinol-carboxylic acid, CBN: Cannabinol, CBD: Cannabidiol.
Introduction:
For decades, veterinarians in clinical practice have known about the abnormal sensitivity of Collies and similar breeds to certain deworming medications. When ivermectin was introduced in the 1980’s, it was hailed as a miracle drug. However, veterinarians soon discovered that the recommended dose would be safe and effective in most breeds, yet deadly in others. As a result, and due to a lack of understanding for the reason behind this scenario, the old adage, ‘white feet, don’t treat’ was used by many practitioners. However, simple appearance alone was not a reliable indicator for predicting adverse reactions. It was long suspected that these ‘ivermectin-sensitive Collies’ had a hereditary condition; however it wasn’t until 2001, that the underlying cause behind the unique idiosyncrasy in these breeds was elucidated. Veterinarian and pharmacologist Katrina Mealey, and a team of researchers at Washington State University, finally discovered the underlying cause of this problem. They discovered a blip in a gene called MDR1 that predisposes herding breeds to adverse, sometimes violent reactions to ivermectin.1 The team published a paper with their groundbreaking findings in Pharmacogenetics that same year.1A Since then, further progress has been made concerning this genetic anomaly, its implications, and the breeds affected. The same group then created a simple buccal swab test to determine if an individual is affected. It has become so clinically significant, that in 2004, WSU patented the test.1
What is MDR1? The Science Behind the Discovery:
Due to the suspicion that the issue was hereditary, the initial approach in understanding the sensitivity of Collies to this drug utilized, in part, theories behind the science of pharmacogenetics, the study of drug response or drug behavior based on an individual’s genetic makeup.7
Many research groups were attempting to discover the underlying cause. One group discovered that the ‘ivermectin-sensitive Collies’ showed highly increased ivermectin accumulation in the brain, suggesting that the protective function of the blood-brain-barrier was defective. This led to the hypothesis that these dogs were analogous to MDR1 knockout mice and had a deficiency in the canine MDR1 gene.
MDR signifies ‘Multi Drug Resistance.’ The MDR1 (also labeled as the ABCB1) gene encodes P-glycoprotein, a drug transporter that plays a key role in drug disposition in multiple species.2 The MDR1 gene exists in all mammals analyzed to date, including the dog.4
Researchers then began to clone and sequence this gene in order to identify the defect, considering the sensitivity to be a ‘genetically determined drug susceptibility.’ It was finally revealed by Mealey et al. when they were the first to identify a 4-bp (base pair) deletion mutation in the MDR1 gene of an ivermectin-sensitive Collie. This MDR1 deletion produces a frame shift, generating several stop codons that prematurely terminate P-glycoprotein synthesis.1A The end-result is a severely truncated, non-functional protein.4
Thus, the WSU research team is credited with the discovery of the MDR1 polymorphism in herding breed dogs, including Collies and Australian shepherds, and this has been demonstrated to be the cause of ivermectin sensitivity in these breeds,8 as well as sensitivity to other classes of drugs.
The Significance of MDR1 and P-glycoprotein:
To appreciate the significance of this discovery, it is critical to understand the mechanisms of drug transporter systems and how they are controlled, specifically P-glycoprotein.
For many drugs used in veterinary practice, plasma and tissue concentrations are highly dependent on the activity of drug transporters. These transporters are large transmembrane proteins that function as either drug efflux or uptake pumps.9 The transporters are highly expressed on the surface of tissues that are responsible for drug absorption, metabolism, and excretion, such as liver, intestinal lumen, biliary canaliculi, and renal tubular epithelium. In addition, these transporters are also expressed on the endothelium of “sanctuary” or protected sites, including brain, retina, testes, and placenta.9
The drug transporter protein at the heart of the Collie sensitivity issue is part of what is known as the ABC (ATP-binding cassette) “superfamily.” There are more than 40 members of the ABC protein superfamily, which use ATP to transport substrates across biological membranes (often against steep concentration gradients). Substrates for ABC transporters include ions, peptides, hormones, conjugated metabolites, and xenobiotics, including drugs.9
It is theorized that due to their ability to prevent drug absorption, enhance drug excretion, and prevent drug entry into specialized tissues, ABC drug transporters likely provide a protective role by decreasing exposure to potentially toxic substances.
Only 3 members of the ABC transporter superfamily are known to transport drug molecules as their substrates, and P-glycoprotein (P-gp) is one of them.9 P-gp is considered an efflux pump. More than 20 therapeutic drugs are known substrates of P-glycoprotein.10
The crux of the issue is that this essential P-glycoprotein is encoded by the ABCB1 gene, more commonly known as the MDR1 gene. Therefore, it is not surprising that deficient ABC transporter function, such as what occurs in individuals with this MDR1 genetic defect, can result in significantly enhanced exposure to substrate drugs, and that these animals may experience extreme sensitivity when exposed to drugs that are substrates for the defective transporter, P-gp.9
Figure 1
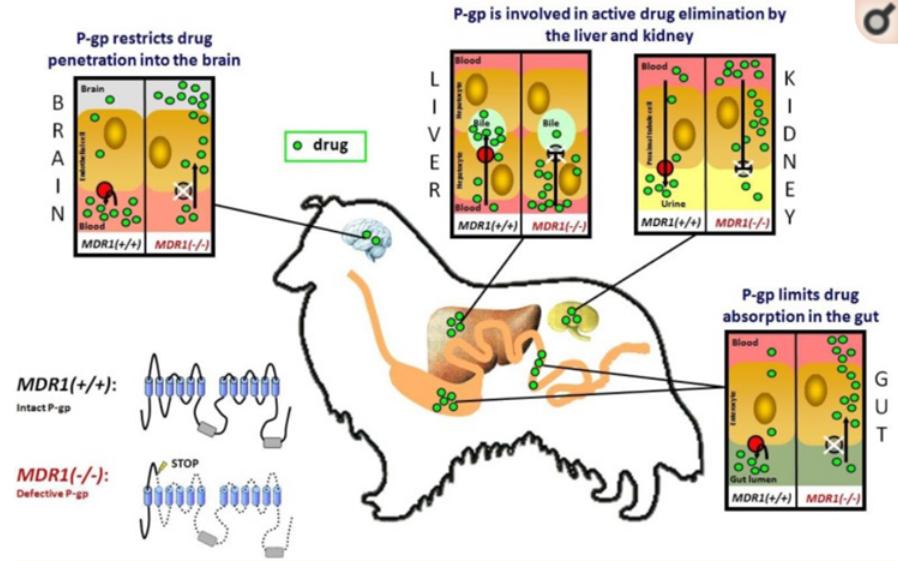
The role of P-gp in drug disposition. P-glycoprotein (shown in red) is an ATP-driven efflux transporter which pumps its substrates out of the cell. The intact P-gp limits drug entry into the organism after oral administration, promotes drug elimination into bile and urine, and restricts drug penetration across the blood-brain barrier. In MDR1(-/-) dogs which do not express a functional P-gp, enteral drug absorption is enhanced, biliary and urinary drug elimination is reduced, and the permeation of blood-tissue barriers is increased at the blood-brain barrier, blood-testis barrier and blood-placenta barrier. As a consequence, P-gp transported drugs can cause an increase in adverse effects in these dogs. This particularly applies to macrocyclic lactones, which would normally be efficiently transported by P-gp.
Source:4 Geyer and Janko: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3419875/
What does this mean clinically?
What this means in practical terms, is that clinicians need to carefully consider the potential impact when using drugs that utilize this transporter system or are substrates of P-gp, in patients that may be affected by this mutation. It is prudent to consider testing for the mutation if the patient is, or is suspected to be, one of the breeds listed below:
Which breeds are affected?2,6
- Australian Shepherd
- Border Collie
- Collie
- English Shepherd
- German Shepherd
- Long-Haired Whippet
- McNab Shepherd
- Miniature Australian Shepherd
- Herding Mixed Breed
- Old English Sheepdog
- Shetland Sheepdog
- Silken Windhound
What does this mean for Pharmaceutical use in these patients?
There are many classes of drugs that individuals with the MDR1 genetic mutation may be sensitive to. The list below reports those pharmaceuticals that have been reported to be of concern in canine patients.
Dogs may be sensitive to:2,3,5,6
- Antibiotics such as erythromycin, doxycycline*and rifampin
- Anti-cancer drugs such as doxorubicin, vinblastine, vincristine, actinomycin D, vinorelbine, docetaxel, and paclitaxel
- Anti-emetic drugs such as ondansetron
- Emetics such as apomorphine
- Anti-diarrheal drugs such as loperamide (Imodium®)
- Certain parasite-control products such as ivermectin (in high doses), milbemycin, moxidectin, doramectin, eprinomectin, selamectin, and emodepside.
- Pain medications such as butorphanol
- Tranquilizers/sedatives such as acepromazine
- Immunosuppressive agents such as cyclosporine*
- Cardiac medications such as digoxin*
*May be safely tolerated by dogs with MDR1 mutation.2 These drugs are part of a large group of drugs that are known substrates of P-gp, yet have not been reported to cause toxicity in MDR1 affected dogs at therapeutic/recommended doses, when compared to normal dogs. 5
In addition, there are several drugs that, on their own, can inhibit P-glycoprotein and become problematic, especially when used in combination with the above…in all patients, with or without the mutation. 2,11
Drugs that also inhibit P-glycoprotein:2,9
- Ketoconazole
- Spinosad
- Cyclosporine
Will Cannabis Adversely Affect these Patients? Is there any scientific evidence?
Where does cannabis fit in this scenario? Are cannabinoids substrates for P-gp? Are patients with this mutation more sensitive to cannabis-derived medications?
These are important questions. This author is not aware of any research specifically investigating cannabinoids in canines with this mutation, so there is much work that needs to be done to increase our understanding, however, there are some research results to help guide the decision-making process.
An in-vitro study in 200612 attempted to investigate the possible interaction of P-gp with each of four major cannabis constituents: delta-9-tetrahydrocannabinol (THC), a metabolite of THC called 11-nor-delta-9-tetrahydrocannabinol-carboxylic acid (THC-COOH), cannabinol (CBN), and cannabidiol (CBD). Their results suggested that all four cannabinoids, especially THC-COOH and CBN, are potential P-gp substrates, which implies that their pharmacokinetics and pharmacodynamics may be affected by different gene types of MDR1 or coadministration of P-gp inhibitors. They also concluded that CBD, in a concentration-dependent manner, exhibits a potent P-gp inhibitory effect, suggesting that CBD could potentially influence the absorption and disposition of other co-administered compounds that are P-gp substrates. The authors noted that the molecular mechanism of P-gp inhibition by CBD could not be clearly revealed, and requires further study using animal models.12
Another paper from 2019,13 a review of medical cannabis and potential drug interactions, concluded that cannabinoids bind to many membrane transporters, including those in the ATP-binding cassette superfamily, breast cancer-resistant protein (BCRP) and Glycoprotein P (P-gp). The authors noted that interactions of cannabinoids with P-gp have been reported in preclinical studies and that the duration of cannabinoids exposure affects the expression of P-gp, with downregulation in chronic exposure and upregulation in short exposure. The authors did note that the concentrations of cannabinoids used to determine these effects were higher than commonly seen in regular human cannabis smokers.13
In a 2012 animal study,14 researchers tested the effects of THC in different populations of mice. They aimed to show that mice devoid of 2 different ABC transporters (ABCB1 (codes for P-gp) and ABCB2 (codes for BCRP)) retain higher brain THC levels and are more sensitive to cannabinoid-induced hypothermia than wild-type (WT) mice. They demonstrated this fact in both populations of ABC transporter-deficient mice, and concluded that their results showed both P-gp and BCRP prolong the brain disposition and hypothermic effects of THC, and offered a novel mechanism for genetic vulnerability to the psychoactive effects of cannabis. Further, they noted that their data implied that THC may have significantly lower affinity for P-gp than BCRP. Nevertheless, the mice devoid of either of these drug transporters exhibited delayed elimination of THC from the brain and thus were more sensitive to hypothermia promoted by THC, and that this may have functional consequences for other CNS-mediated actions of THC.14
These, and other studies, are slowly increasing our general knowledge of cannabinoids and their interaction with this vital drug transport system.
Practical Steps for Considering Cannabis in these Affected Dogs.
How can this information be applied to the practice of veterinary medicine? Can clinicians consider using cannabis safely in these affected patients?
It is reasonable to assume from the above evidence, that various cannabinoids have the potential for enhanced effects in patients suffering from MDR1 genetic mutations, as compared to normal dogs. For practical purposes, consider several important factors:
- Genotype of the individual (Homozygous vs Heterozygous)
This factor is crucial because it translates directly to the level of dysfunction in the patient. Dogs that are homozygous for this mutation have no P-glycoprotein function, whereas heterozygotes have partial P-glycoprotein function.2 Genetic testing is the only way to determine the patient’s genotype.
Homozygous = mutant/mutant: Affected animals have 2 copies of the mutant allele and always pass 1 copy of the defective gene to offspring. Homozygotes are more likely to have severe (life-threatening) adverse drug reactions.
Heterozygous = mutant/normal: Affected animals have 1 copy of the mutant allele and 1 copy of the normal allele and have a 50% chance of passing the defective gene to offspring. Heterozygotes tend to have less severe adverse drug reactions as compared with homozygotes.2
- Dosage
The common mantra in cannabinoid medicine of “Start Low and Go Slow” is especially important in these situations. Based on recommendations for dose reductions in drugs that are known to cause toxicities in these patients,11 it may be prudent to consider following the same model when formulating a treatment plan with cannabinoids. Dose calculations of many suspect drugs are reduced 30-50% for homozygous dogs and 25% in heterozygous dogs, as compared to normal. In other words, “Start lower and go slower…”
- Co administered medications
Avoid use of other, non-cannabinoid, substances that may be P-gp inhibitors.
- The cannabinoid(s) medication being considered
The active drug/ingredient(s) of any medication being considered for use in these patients can fall into one or more of several different categories.
- Those that are P-gp substrates and have proven to be problematic, i.e. acepromazine
- Those that are P-gp substrates and have the potential to be problematic, but their status is unknown due to lack of research or widespread use, i.e. cannabis
- Those that are substrates of P-gp and yet, do not cause clinical toxicities, i.e. penicillin
Currently, cannabis would appear to fit into category “b.” However, there are many pharmaceuticals, such as cephalosporins, penicillins, tetracyclines, antihistamines, and β-adrenergic antagonists, that fall into category “c.”2 This is because they have one or more characteristics that allow for safe use in animals with P-glycoprotein dysfunction, including:
- Wide therapeutic margin
- Minimal neurologic toxicity
- Alternate drug clearance mechanisms
- The ability to be pumped by a different drug transporter2
Time will tell if cannabis as a whole, and the individual cannabinoids, may eventually fit into category “c” due to having one or more of these characteristics.
Conclusion and Final thoughts.
While therapeutic doses of cannabinoids are generally considered to have a wide margin of safety, an increased risk of adverse events is expected in those with genetic, or other predispositions to drug toxicities.
The lack of targeted research into this dilemma is noteworthy, since species specific variations may be relevant. This leads to the reliance on anecdotal information.
On the purely conservative side, when asked specifically about cannabis use in these patients, the leading expert, Dr. Mealey, replied that she has had anecdotal reports of CNS depression in dogs with the MDR1 mutation that have been exposed to cannabinoids, and for that reason, her general recommendation is to avoid them in these dogs.15 However, no specific details were provided as to dose, cannabinoids, or patient specific information relating to these reports.
Conversely, this author is aware of at least one dog with confirmed MDR1 mutation, under treatment with cannabinoids that experienced no adverse effects. Perhaps it requires a much larger dose of CBD or other cannabinoids to inhibit p-glycoprotein in vivo.
There is one more point worth noting. In recent years, there has been an increase in reports of accidental cannabis intoxications by dogs, coinciding with widespread legalization. One would think that due to this fact, combined with the popularity of these breeds amongst American pet owners, we would be seeing some statistical evidence of herding breeds suffering from disproportionately higher incidences of toxicity resulting from cannabis use, either therapeutic or accidental. To this author’s knowledge, this is not the case. In fact, there is little breed specific data available in this regard.
While all of this information may ask more questions than it actually answers, one thing is certain…research in this subject is in its infancy, and there is much work to be done!
The available conflicting information leaves the practitioner in a quandary. Therefore, until we have more relevant data, the only prudent solution is to be cautious, to carefully consider all aspects of the case, and utilize practical steps, including those listed above, when formulating an individualized treatment plan involving cannabis in patients who may be more vulnerable, due to MDR1 genetic mutation.
References:
1.https://news.wsu.edu/2013/07/08/researcher-recognized-for-drug-reaction-discovery-in-dogs/
1A. Mealey, K. L., Bentjen, S. A., Gay, J. M., & Cantor, G. H. (2001). Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics, 11(8), 727–733. https://doi.org/10.1097/00008571-200111000-00012 https://pubmed.ncbi.nlm.nih.gov/11692082/
2. Cliniciansbrief.com, May 2016. Ask the Expert: MDR1 Gene Mutations and Drug Therapy. Katrina Mealey, DVM, PhD, DACVIM, DACVCP. Washington State University. https://vcpl.vetmed.wsu.edu/docs/librariesprovider17/default-document-library/ask_-mdr1-gene-mutations-may-2016.pdf?sfvrsn=de7acb38_2
3.https://www.animalgenetics.us/canine/Genetic_Disease/MDR1.asp
4. Geyer, J., & Janko, C. (2012). Treatment of MDR1 mutant dogs with macrocyclic lactones. Current pharmaceutical biotechnology, 13(6), 969–986. https://doi.org/10.2174/138920112800399301 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3419875/
5.https://vcpl.vetmed.wsu.edu/problem-drugs
7.https://todaysveterinarynurse.com/articles/mdr1-genetic-testing-what-you-need-to-know/
8. Mealey K. L. (2004). Therapeutic implications of the MDR-1 gene. Journal of veterinary pharmacology and therapeutics, 27(5), 257–264. https://doi.org/10.1111/j.1365-2885.2004.00607.x https://pubmed.ncbi.nlm.nih.gov/15500562/
9. Mealey, Katrina L.(2013) Adverse Drug Reactions in Veterinary Patients Associated with Drug Transporters. Veterinary Clinics: Small Animal Practice, Volume 43, Issue 5, 1067 – 1078. http://dx.doi.org/10.1016/j.cvsm.2013.04.004 https://www.vetsmall.theclinics.com/article/S0195-5616(13)00109-5/pdf
10. Neff, Mark & Robertson, Kathryn & Wong, Aaron & Safra, Noa & Broman, Karl & Slatkin, Montgomery & Mealey, Katrina & Pedersen, Niels. (2004). Breed distribution and history of canine mdr1-1Δ, a pharmacogenetic mutation that marks the emergence of breeds from the collie lineage. Proceedings of the National Academy of Sciences of the United States of America. 101. 11725-30. 10.1073/pnas.0402374101. https://www.pnas.org/content/101/32/11725 https://www.researchgate.net/publication/8420150_Breed_distribution_and_history_of_canine_mdr1-1D_a_pharmacogenetic_mutation_that_marks_the_emergence_of_breeds_from_the_collie_lineage
11. https://cliniciansbrief.com/article/how-should-i-treat-dogs-cats-mdr1-mutation
12. Zhu, H. J., Wang, J. S., Markowitz, J. S., Donovan, J. L., Gibson, B. B., Gefroh, H. A., & Devane, C. L. (2006). Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. The Journal of pharmacology and experimental therapeutics, 317(2), 850–857. https://doi.org/10.1124/jpet.105.098541 https://www.theroc.us/researchlibrary/Characterization%20of%20P-glycoprotein%20Inhibition%20by%20Major%20Cannabinoids%20from%20Marijuana.pdf
13. Alsherbiny MA, Li CG. Medicinal Cannabis-Potential Drug Interactions. Medicines (Basel). 2018;6(1):3. Published 2018 Dec 23. doi:10.3390/medicines6010003 https://pubmed.ncbi.nlm.nih.gov/30583596/
14. Spiro AS, Wong A, Boucher AA, Arnold JC (2012) Enhanced Brain Disposition and Effects of Δ9-Tetrahydrocannabinol in P-Glycoprotein and Breast Cancer Resistance Protein Knockout Mice. PLoS ONE 7(4): e35937. https://doi.org/10.1371/journal.pone.0035937 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0035937
15. Katrina Mealey, DVM, PhD, DACVIM, DACVCP. Personal communication.

